By: Sikandar Khan Sherwani, Faculty: FUUAST university, Karachi,
President- Elect Medical Microbiology Association of Pakistan
The theme for World Ozone Day 2018 Keep Cool and Carry On: The Montreal Protocol. The theme is appealing and no doubt motivational as many countries are seriously taking interest to comply the precautionary measures to combat this nuisance.
The OZONE LAYER
The ozone layer was discovered in 1913 by the French physicists Charles Fabry and Henri Buisson. .The ozone layer, a protective shield of colourless gas, that basically have a purpose to safeguard the Earth from the harmful and critical rays of the sun making this earth, a safer place for the inhabitants.
Composition of Ozone Layer:
The ozone layer possesses less than 10 parts per million of ozone, on the other hand, the average ozone concentration in Earth’s atmosphere as a whole is about 0.3 parts per million. The ozone layer primarily exists in the lower portion of the stratosphere, from approximately 20 to 30 kilometers (12 to 19 mile) above Earth. The ozone layer absorbs around 97 to 99 % of the Sun’s medium-frequency ultraviolet light (from about 200 nm to 315 nm wavelength), which otherwise would potentially damage different forms of life near the surface of earth.
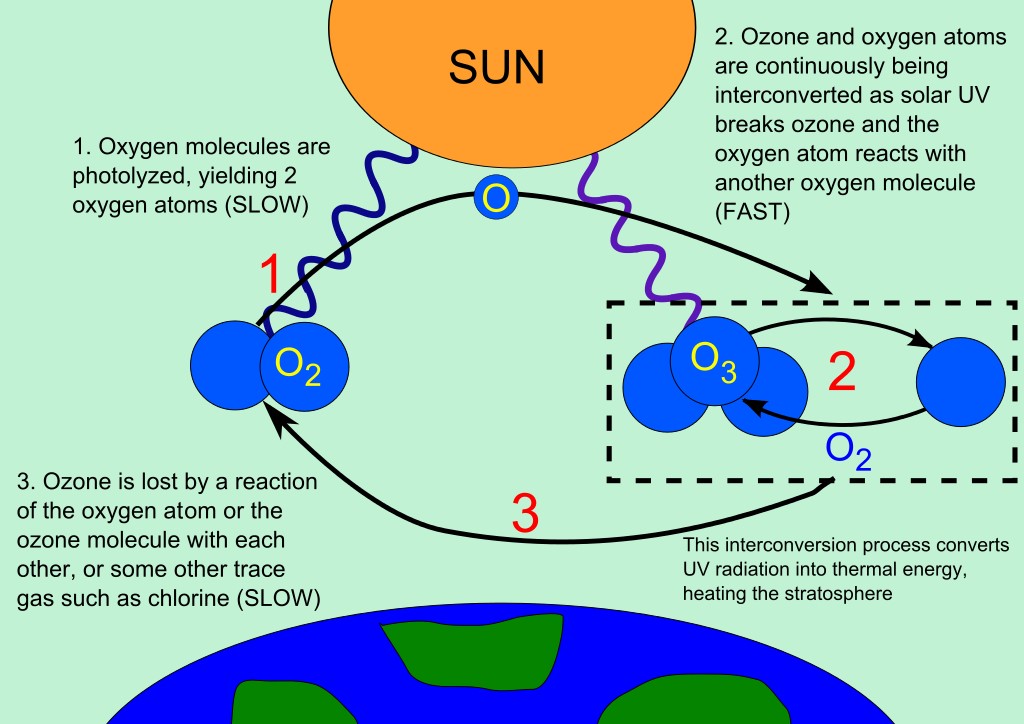
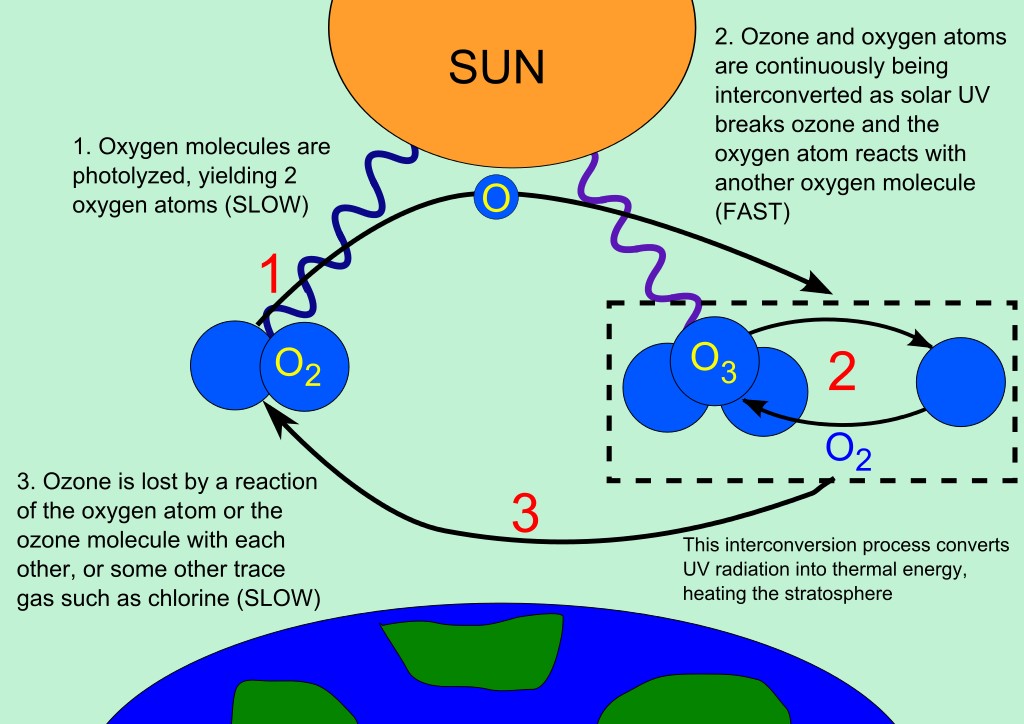
Fig : the picture shows Ozone-oxygen cycle in the ozone layer.
Factors for the depletion of Ozone Layer:
The main reason that the ozone layer can be depleted as a result of certain free radical catalysts. Among them the most noticeable are nitric oxide (NO), nitrous oxide (N2O), hydroxyl (OH), atomic chlorine (Cl), and atomic bromine (Br). It has been noted since many years that few organo compounds (man made) have contributed greatly in the depletion of ozone layer ,like chlorofluorocarbons (CFCs) and bromofluorocarbons. The most alarming situation is that such compounds exist form 20-120 years without being degraded and finally playing a role to reach to stratosphere. These highly stable compounds are capable of surviving and reach to the to the atmospheric surface , where radicals of Cl and Bromine are liberated by the action of ultraviolet light.
Many scientists have strongly advised that wise and careful uses of ozone depleting substances will not only contributing in reducing global warming bit also have a profound impact on the health of mankind. Ozone in the Earth’s stratosphere is generated by ultraviolet light that hits basically ordinary oxygen molecules containing two oxygen atoms (O2), therefor, splitting them into individual oxygen atoms (atomic oxygen); the atomic oxygen then combines with unbroken O2 to develop ozone, O3. The ozone molecule is unstable and when ultraviolet light hits ozone then splits into a molecule of O2 and an individual atom of oxygen, this process commonly called the ozone-oxygen cycle. Chemically, this can be described as:
O2 + ℎνuv → 2 O
O + O2 ↔ O3
Threatened areas :
The ozone layer above the Antarctic has been particularly disturbed owing to the pollution since the mid-1980s. This region has got low temperatures that in turn increases the conversion of CFCs to chlorine. In the southern spring and summer, when the sun shines for long periods of the day, chlorine reacts with ultraviolet rays, destroying ozone on huge grounds around 65 percent. Therefore, some people has now characteristically marked as the “ozone hole.” In other regions, the ozone layer has deteriorated by about 20 percent.
Human health at risk:
Moreover, these UV rays cause serious harm to living beings and environment, animals and plants. Exposure to these rays can cause skin related issues and problems as high as skin cancer. Environmentalists suggest also that these rays can increase the probability of the spread of infectious diseases. They also affect the eyes and can cause vision problems. Plants and animals are also the victims of UV rays exposure and it affects the food chain ultimately food web seriously. In short, the depletion of the ozone layer results in an imbalanced ecosystem.
- Skin cancer:Exposure to ultraviolet rays poses an increased risk of developing several types of skin cancers, including malignant melanoma, and basal and squamous cell carcinoma.
- Eye damage:Direct exposure to UV radiations can result in photokeratitis (snow blindness), and cataracts.
- Immune system damage:Effects of UV rays include impairment of the immune system. Increased exposure to UV rays weakens the response of the immune system.
- Aging of skin:Constant exposure to UV radiation can cause photo allergy, which results in the outbreak of rashes in fair-skinned people.
- Other effects:Ozone chemicals can cause difficulty in breathing, chest pain, throat irritation, and hamper lung functioning.
Steps to save earth:
- Few general routine steps can help us greatly to save our earth.
- Plant more tress.
- Maintain properly the vehicles from the emission.
- Walk more, drive less
- Introduce eco friendly products etc.
- Recycle and reuse stuff
- Harness renewable energy